On December 17, 2025, the Official Journal of the EU published the guidance (C/2025/6721) of (EU) 2024/3190. Regulation (EU) 2024/3190 prohibits the use of bisphenol A in specific food contact materials and also restricts other harmful bisphenols and bisphenol derivatives. This guideline is presented in a Q&A format and details the scope of application of regulation (EU) 2024/3190, the list of prohibited bisphenols and bisphenol derivatives substances, compliance testing, market placement and the transitional provisions.
The main contents of the guidance are as follows:
Scope
1.Food-contact paper and board, recycled materials, enamel products, and materials and articles intended for contact with pet food are excluded from the scope of the (EU) 2024/3190.
2.The external parts of FCMs, if they can reasonably be expected to be brought into contact with food or to transfer their constituents to food under normal or foreseeable conditions of use, Regulation (EU) 2024/3190 applies.
3.Starting materials, intermediate materials and finished food contact materials and articles, which are manufactured from the materials specified in Article 1(2) of Regulation (EU) 2024/3190 are therefore subject to that Regulation.
4.If the pipes are permanently affixed to vats and tanks, and the resulting self-supporting material or article in its entirety, has a capacity exceeding 1 000 litres, they are considered to fall under the scope of the derogation. Pipes with a high surface area to volume ratio and small pipes do not fall under the scope of the derogation.
List of prohibited bisphenols and bisphenol derivatives
● The use of Bisphenol A (BPA) and its salts in the manufacture of food contact materials and articles is prohibited, by way of derogation for specific applications set out in Annex II. Bisphenol-A diglycidyl etherr (BADGE) (CAS No 1675-54-3) can be used in the manufacture of food contact materials and articles; However, such articles shall not contain any residual BPA.
● The use of bisphenols and bisphenol derivatives listed in Part 3 of Annex VI to Regulation (EC) No 1272/2008, due to their harmonised classification as category 1A or 1B ‘mutagenic’, ‘carcinogenic’, ‘toxic to reproduction’ or category 1 ‘endocrine disrupting’ for human health’, is prohibited in the manufacture of food contact materials and articles (the list of substances is provided in the table below)
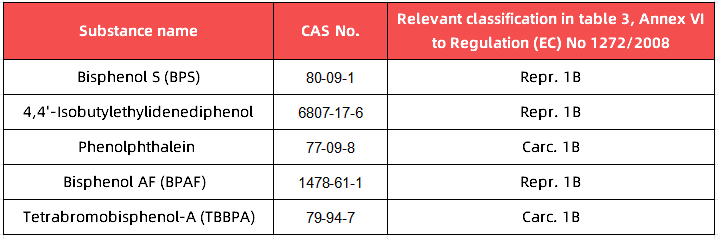
It can also be noted that, at the time of the adoption of the guidance, an ECHA opinion was published on 17 September 2024 concerning a proposed harmonised classification (Repr. 1B) for bisphenol F (4,4'-methylenediphenol) (CAS no. 620-92-8).This means that, when substances with harmonised classification as category 1A or 1B CMR or category 1 ED are added to Part 3 of Annex VI to Regulation (EC) 1272/2008, their use will also be prohibited by Regulation (EU) 2024/3190.
Compliance testing
● Supporting documentation that accompanies the DoC can demonstrate that BPA has not been used in the manufacture of the FCM, including, for example, a list of monomers or starting substances that have been used. In such situations, further verification of compliance by testing is at the discretion of the business operator.
● For food contact materials and articles in which BPA may be used in accordance with Annex II of the Regulation, it will be necessary to demonstrate that migration of BPA does not occur above the detection limit (with a detection limit of 1 μg/kg).
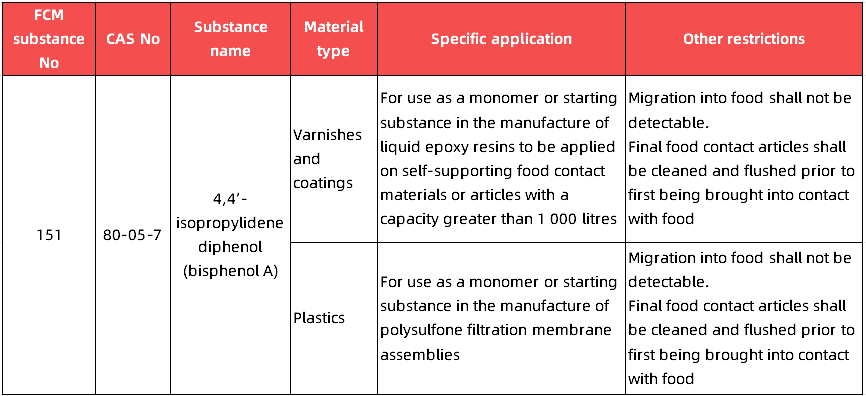
● Food contact materials and articles that have been manufactured using another bisphenol or bisphenol derivative shall not contain any residual BPA (with a detection limit of 1 μg/kg).
Declaration of compliance
All materials specified in Article 1(2) of Regulation (EU) 2024/3190, and including multi-material articles, must be accompanied by a DoC, even if no BPA has been used in the manufacturing process. This means, for example, that printing inks and other materials covered by the scope of the Regulation, and which are intended as food contact materials and/ or to form part of a final food contact article, require a DoC. The resulting multi-material or multi-layer final food contact articles must also be accompanied by a DoC fulfilling the information requirements set out in Annex III.
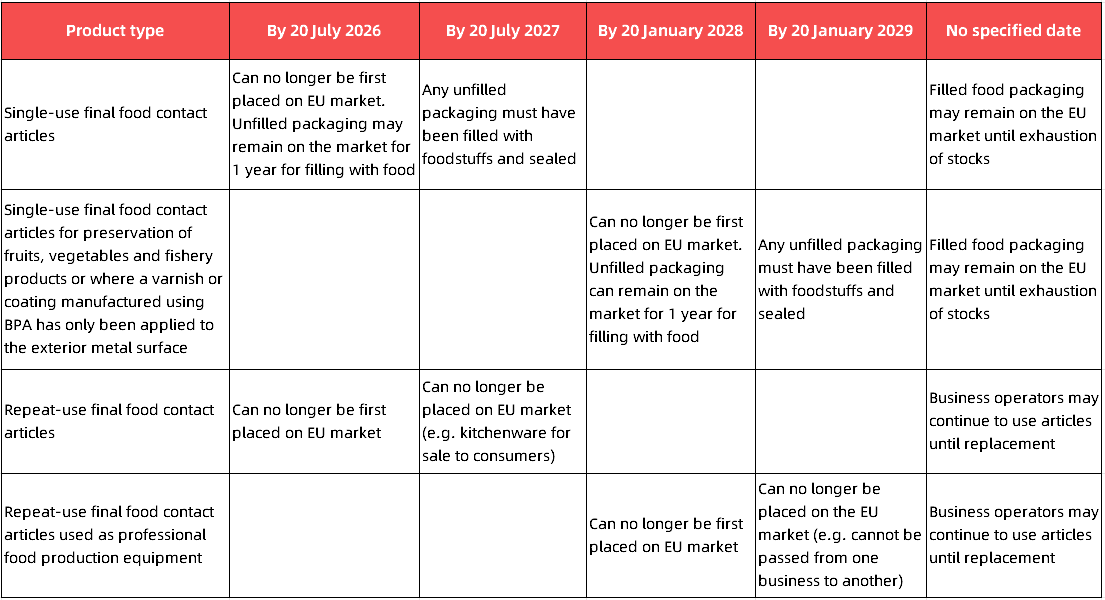
Transitional provisions
The main requirements during the transition period are as follows:
CTT Reminder:
The guidance provides a clear roadmap for enterprises to achieve compliance. It offers detailed clarifications on the scope of Regulation (EU) 2024/3190 and the regulated substances, explicitly defining the stringent detection limit of 1 μg/kg. Enterprises should thoroughly review their products and supply chains based on this guidance and pay close attention to the transitional periods applicable to different products to ensure continued market access. CTT can provide enterprises with one-stop comprehensive solutions for consulting, testing and training. For more detailed information, please contact us.
Link:https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:C_202506721
