On July 30, 2024, the European Commission submitted a notification with the number G/TBT/N/EU/1079 to the WTO's TBT. It is planned to update Annex XVII of the REACH Regulation, adding restrictions on N,N-dimethylformamide (DMAC) and 1-ethyl-2-pyrrolidinone (NEP). Stakeholders may provide feedback within 60 days after the publication of the notice.
The draft proposes to add the following to Annex XVII:
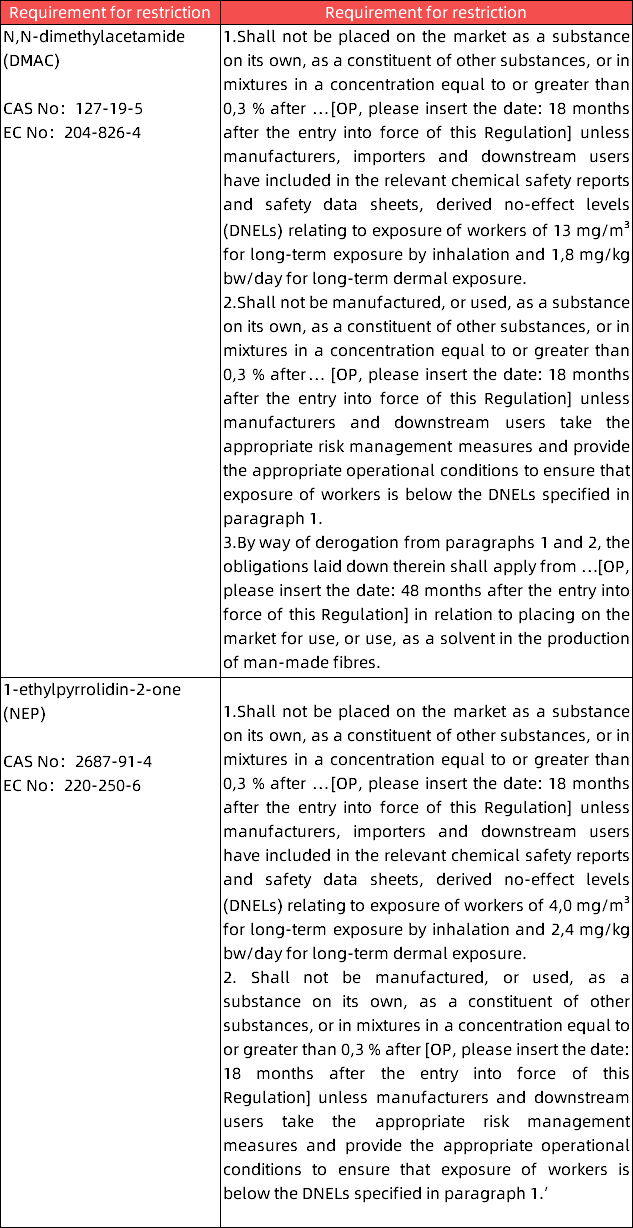
About DMAC and NEP:
(1)N,N-dimethylacetamide (DMAC) and 1-ethylpyrrolidin-2 one (NEP) are dipolar aprotic solvents. DMAC is listed in Part 3 of Annex VI to Regulation (EC) No 1272/20082 as toxic to reproduction category 1B based on developmental toxicity and as acute toxic category 4. NEP is listed in Part 3 of Annex VI to Regulation (EC) No 1272/2008 as toxic to reproduction category 1B based on developmental toxicity.
(2)DMAC and NEP are used in industrial settings and by professionals as solvents in the formulation of mixtures, for example in agrochemicals, pharmaceuticals and fine chemicals. DMAC is also used as a solvent in coatings and is extensively used in the production of man-made fibres and films and during the production of polyamide imide enamels (varnishes) used for electrical wire insulation. NEP is applied in cleaning agents and as a binder and a release agent. NEP is also used in oil field drilling and production operation processes, in functional fluids, in polymer processing, in water treatment, as an excipient in agrochemicals and in road and construction applications. Both substances are used as a laboratory agent.
